g mol of water|what is the weight of 12 moles water : Pilipinas More information from the unit converter. How many moles Water in 1 grams? The answer is 0.055508435061792. We assume you are converting between moles Water and .
Resultado da Kinechan corona com ted blondeliberal fodendo em festa caseira de swing, com binho ted. Xxx-hd. Vídeos Relacionados. HD. 39 min. Kinechan carona ted . HD. 30 min. Mc pipokinha na corona do ted . HD. 15 min. Carona do ted com a kinechan . HD. 11 min. Corona com ted comedo . HD. 5 min. .
0 · what is the weight of 12 moles water
1 · weight of 1 mole water
2 · volume of 1 molecule water
3 · moles of water in 1 litre
4 · mass of 1 molecule water
5 · mass of 1 mole water
6 · how much does a mole of water weight
7 · how many moles are in 40.0 grams of water
8 · More
Resultado da Our free Ask AI Answer Engine enables users to ask questions in a .
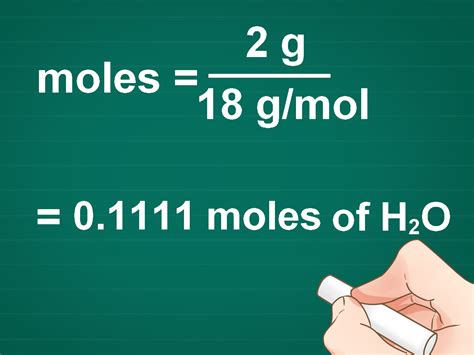
g mol of water*******To calculate this result: Calculate the molar mass of water, which is two hydrogen atoms' and one oxygen atom's molar masses combined: (2 × 1.008 g/mol) + 15.999 g/mol = 18.015 g/mol. Divide the mass of your sample by the molar mass: 100 g / 18.015 g/mol = 5.551 mol. This is the number of moles in 100 g . See more
This article explains what is Grams to Moles Calculator and how it works with its formula. It also mentions that the tool helps users find the . See more
To estimate the number of moles, divide mass (in g) by the substance's molar mass (g/mol). A mole is an SI unit for measuring amount, equal to 6.02214085774 × 10²³ atoms or molecules. See moreg mol of waterThe grams to moles calculator helps you convert grams of a substance into the number of moles. It is user-friendly and straightforward to use. You can also find the total number of . See moreMeasure mass in g, find substance's molar mass, divide mass by its molar mass = number of mol in sample. See more
More information from the unit converter. How many moles Water in 1 grams? The answer is 0.055508435061792. We assume you are converting between moles Water and .
Calculate the molar mass of Water in grams per mole or search for a chemical formula or substance. Molecular weight of Water. Water molecular weight. Molar mass of H2O = . Volume = mass / density. Volume of 1 mole of water = (18 g) / (1 g/ml) Volume of 1 mole of water = 18 ml. One mole of water is about 18 milliliters. This is the volume of a few drops of water, 3.65 . This means 1 mole of hydrogen weighs 1.0079 grams and 1 mole of oxygen weighs 15.9994 grams. Therefore, water would weigh: weight of water = 2 (1.0079) g + .
Solution: Find out the molar mass of the substance (hint: you can use Molar mass of the substance alone to calculate molar mass). The molar mass of KClO3 is 122.548 g/mol. .
Referring to the periodic table, the atomic mass of K is 39.10 amu, and so its molar mass is 39.10 g/mol. The given mass of K (4.7 g) is a bit more than one-tenth the .what is the weight of 12 moles water Water has a molarity of 55.5 M. 1 liter of water weighs 1000 g, and, as molarity is the number of moles per liter; finding the molarity of water is the same as .
How many moles of H 2 O are present in 240.0 g of water (about the mass of a cup of water)? Solution. Use the molar mass of H 2 O as a conversion factor from mass to .
This number of particles is Avogadro’s Number. It is approximately 6.02x10^23. 6.02x10^23 atoms of carbon make up a mole. 6.02x10 23 teachers of . Volume = mass / density. Volume of 1 mole of water = (18 g) / (1 g/ml) Volume of 1 mole of water = 18 ml. One mole of water is about 18 milliliters. This is the volume of a few drops of water, 3.65 .
The molar mass of any compound is the mass in grams of one mole of that compound. One mole of carbon dioxide molecules has a mass of 44.01g 44.01 g, while one mole of sodium sulfide formula units has a mass of 78.04g 78.04 g. The molar masses are 44.01g/mol 44.01 g/mol and 78.04g/mol 78.04 g/mol respectively. In both cases, that is . Assume we want to dissolve 70.128 grams of salt in 1.5 kg of water. So: moles of NaCl = 70.128 g / (58.44 g/mol) = 1.2 mol. Plug the number of moles and the mass of the solvent into the molality formula. Divide 1.2 mol by 1.5 kg, and you'll find out that the molality of the NaCl solution is 0.8 molal (in standard molality units: 0.8 mol/kg).One mole of Water is composed of 1 mole of Oxygen and two moles of Hydrogen. The mass of oxygen equal to one mole of oxygen is 15.998 grams and the mass of one mole of hydrogen is 1.008 g. If we total up the gram amounts of each element in the water molecule = 15.998g/mol + 2(1.008g/mol) we get the molar mass of water = 18.014g/mol.

Molar Mass of Water. Find the molar mass of water. Do this by looking up the atomic masses of hydrogen and oxygen given on the periodic table. Then add the mass of hydrogen atoms and oxygen atoms in a molecule of water. The mass of hydrogen is 1.008 g/mol and the mass of oxygen is 16.00 g/mol. The mass of one mole of water is:The molar mass of a chemical compound represents the mass of one mole of that substance. For water (H₂O), which consists of hydrogen (H) and oxygen (O), calculating the molar mass involves summing the atomic masses of its constituent atoms. . (H₂O) = (1.008 g/mol × 2) + (16.00 g/mol × 1) Step 6: Calculate - Using the given atomic masses . Referring to the periodic table, the atomic mass of K is 39.10 amu, and so its molar mass is 39.10 g/mol. The given mass of K (4.7 g) is a bit more than one-tenth the molar mass (39.10 g), so a reasonable “ballpark” estimate of the number of moles would be slightly greater than 0.1 mol. The molar amount of a substance may be calculated by .
g mol of water what is the weight of 12 moles waterWe can use the rearranged molarity equation to calculate the moles of NaCl needed for the specified concentration and volume: mol NaCl = [ NaCl] × L of solution = 0.800 mol L × 0.250 L = 0.200 mol NaCl. We can then use the molecular weight of sodium chloride, 58.44 g mol , to convert from moles to grams of NaCl :
Molar concentration, also known as molarity, and can be denoted by the unit M, molar. To prepare 1 L of 0.5 M sodium chloride solution, then, as per the formula, use 29.22 g of sodium chloride (0.5 mol/L * 1L * 58.44 g/mol = 29.22 g). The mass molarity calculator tool calculates the mass of compound required to achieve a specific molar .Water (chemical formula: H2O) is a transparent fluid which forms the world's streams, lakes, oceans and rain, and is the major constituent of the fluids of organisms. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds. Water is a liquid at standard ambient temperature and .
H = 1.01 g/mol O = 16.00 g/mol; H 2 O = 2 + 16 = 18 g/mol (look at the subscript to note there are 2 hydrogen atoms) Use this value to convert the total number of grams of water into moles: (1 mol / 18 g ) * 100 g = 5.56 moles of water Now you have the information needed to calculate mole fraction. X salt = moles salt / (moles salt + moles .Step 1: First, convert the mass of solute to moles using the molar mass of HCl (36.5 g/mol): 22.4 gHCl × 1 molHCl 36.5 gHCl = 0.614mol HCl 22.4 g H C l × 1 m o l H C l 36.5 g H C l = 0.614 m o l H C l. Step 2: Now we can use the definition .
Years active. 2014–present. Mia Khalifa ( / miːə kəˈliːfə /; Arabic: ميا خليفة Miyа̄ Ḵalīfah [mijaː χaliːfa (h)]; born 1993 [1]) is a Lebanese-American media personality and former .
g mol of water|what is the weight of 12 moles water